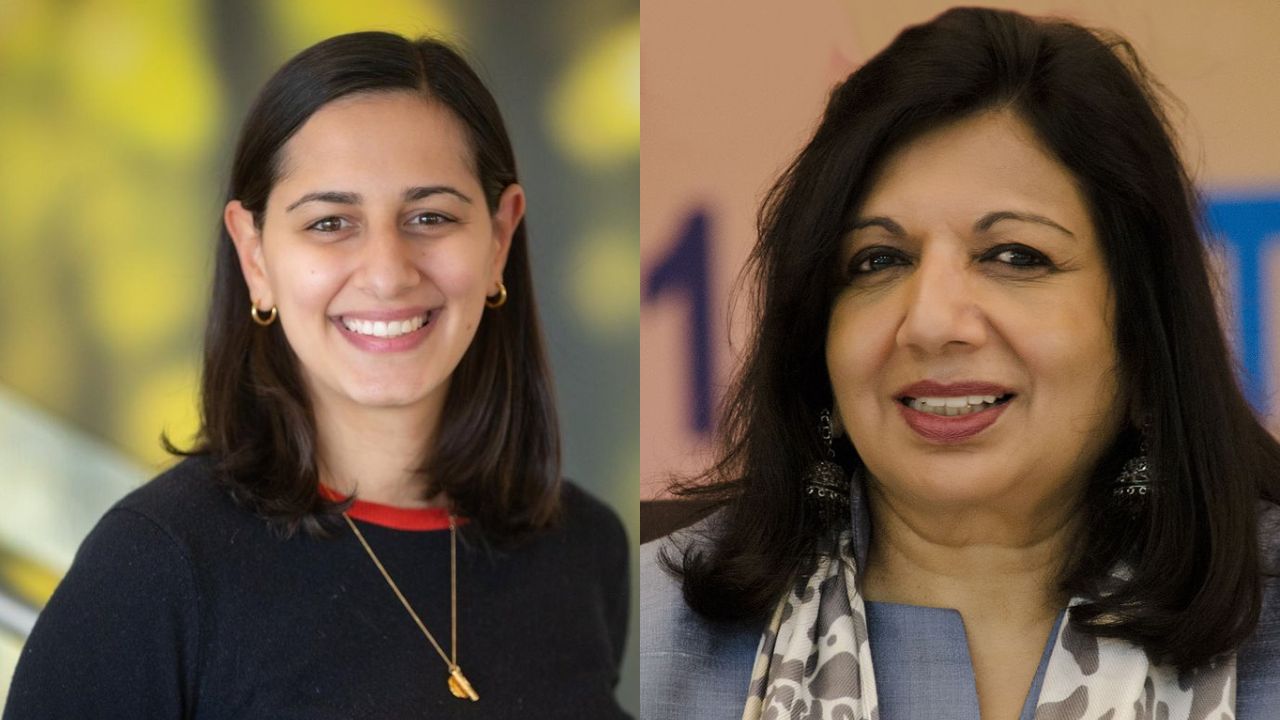Kiran Mazumdar-Shaw, founder and chairperson of Biocon, shared a moment of personal and professional pride on Wednesday, accompanying her niece Claire Mazumdar at the Nasdaq stock exchange in New York City. Claire Mazumdar’s Boston-based biopharmaceutical company, Bicara Therapeutics, made a billion-dollar debut, with both women celebrating the significant milestone by ringing the opening bell.
Mazumdar-Shaw expressed her excitement on social media, writing, “Thrilled to be present at ringing the opening bell for Bicara Therapeutics’ debut on Nasdaq exchange. So very proud of my amazing niece Claire Mazumdar… I am bursting with pride to see Indian innovation being valued in the world’s most respected stock exchange.” Claire Mazumdar, the founding CEO of Bicara, led the company to this momentous occasion, marking a major achievement in her entrepreneurial journey.
Bicara Therapeutics, a clinical-stage biopharmaceutical company, is focused on developing bifunctional therapies aimed at treating patients with solid tumors. The company’s lead drug, ficerafusp alfa, is currently undergoing an early-stage study in the US in combination with Merck’s cancer therapy, Keytruda, to treat head and neck cancers.
The successful IPO marks a new chapter for the company, which plans to use part of the proceeds to fund a mid-to-late stage clinical trial for ficerafusp alfa, expected to commence by late 2024 or early 2025.